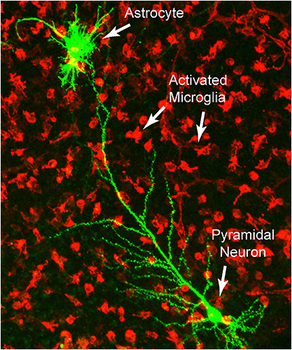
Microglia are the first line of immune defense in the central nervous system, which means microglial activation is an indicator of inflammation. Chronic activation of microglia can lead to excitotoxicity, a mechanism that has been previously proposed for both Gulf War Illness and Autism (Blaylock, "Chronic Microglial Activation and Excitotoxicity Secondary to Excessive Immune Stimulation: Possible Factors in Gulf War Illness and Autism”). The John Hopkins study acts as confirmation that excitotoxicity caused by chronic inflammation is central to autism.
Excitotoxicity has been put forth as a mechanism of ME/CFS by a number of clinicians and researchers, including Drs. Paul Cheney, Jay Goldstein, Morris and Maes, Martin Pall, and, most recently, Jarred Younger.
What are the implications of this study for ME/CFS? As many have stated, it is clear that there is a connection between neuro-inflammation and a number of illnesses that show CNS and immune abnormalities. This study provides anatomical proof of inflammation in the brains of one of the illnesses on the neuro-immune spectrum, which opens the door for badly needed autopsy studies on ME/CFS patients, particularly those with severe cases.
___________________
Brain inflammation a hallmark of autism, large-scale analysis shows
By Shawna Williams
Press Release: Johns Hopkins, December 10, 2014. While many different combinations of genetic traits can cause autism, brains affected by autism share a pattern of ramped-up immune responses and related inflammation, an analysis of data from autopsied human brains reveals.
The study, a collaborative effort between Johns Hopkins and the University of Alabama at Birmingham, included data from 72 autism and control brains. It was published online last week in the journal Nature Communications.
"There are many different ways of getting autism, but we found that they all have the same downstream effect," says Dan Arking, an associate professor in the McKusick-Nathans Institute for Genetic Medicine at the Johns Hopkins University School of Medicine. "What we don't know is whether this immune response is making things better in the short term and worse in the long term."
The causes of autism, also known as autistic spectrum disorder, remain largely unknown and are a frequent research topic for geneticists and neuroscientists. But Arking had noticed that for autism, studies of whether and how much genes were being used—known as gene expression—had thus far involved too little data to draw many useful conclusions. That's because unlike a genetic test, which can be done using nearly any cells in the body, gene expression testing has to be performed on the specific tissue of interest—in this case, brains that could only be obtained through autopsies.
To combat this problem, Arking and his colleagues analyzed gene expression in samples from two different tissue banks, comparing gene expression in people with autism to that in controls without the condition. All told, they analyzed data from 104 brain samples from 72 individuals, the largest data set so far for a study of gene expression in autism.
Previous studies had identified autism-associated abnormalities in cells that support neurons in the brain and spinal cord. In this study, Arking says, the research team was able to narrow in on a specific type of support cell known as a microglial cell, which polices the brain for pathogens and other threats. In the autism brains, the microglia appeared to be perpetually activated, with their genes for inflammation responses turned on.
"This type of inflammation is not well understood, but it highlights the lack of current understanding about how innate immunity controls neural circuits," says Andrew West, an associate professor of neurology at the University of Alabama at Birmingham who was involved in the study.
Arking notes that, given the known genetic contributors to autism, inflammation is unlikely to be its root cause. Rather, he says, "This is a downstream consequence of upstream gene mutation."
The next step, he says, would be to find out whether treating the inflammation could ameliorate symptoms of autism.
Other authors on the study are Simone Gupta, Shannon E. Ellis, Foram N. Ashar, Anna Moes, Joel S. Bader, and Jianan Zhan, all of The Johns Hopkins University. The study was funded by the Simons Foundation and the National Institute of Mental Health.
Journal Reference: Simone Gupta, Shannon E. Ellis, Foram N. Ashar, Anna Moes, Joel S. Bader, Jianan Zhan, Andrew B. West, Dan E. Arking. Transcriptome analysis reveals dysregulation of innate immune response genes and neuronal activity-dependent genes in autism. Nature Communications, 2014; 5: 5748 DOI: 10.1038/ncomms6748

 RSS Feed
RSS Feed
